215x Filetype PDF File size 0.60 MB Source: www.ciaaw.org
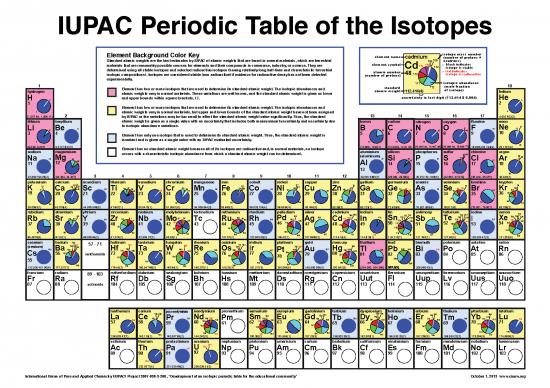
IUPAC Periodic Table of the Isotopes
Element Background Color Key element name isotope mass number
cadmium 108 (number of protons +
Standard atomic weights are the best estimates by IUPAC of atomic weights that are found in normal materials, which are terrestrial 106 neutrons)
116 , black indicates
materials that are reasonably possible sources for elements and their compounds in commerce, industry, or science. They are element symbol 113 116 .
113 114 isotope is stable
determined using all stable isotopes and selected radioactive isotopes (having relatively long half-lives and characteristic terrestrial Cd
,
atomic number 48 . red indicates
isotopic compositions). Isotopes are considered stable (non-radioactive) if evidence for radioactive decay has not been detected (number of protons) 110 isotope is radioactive
experimentally. isotopic abundance
1 111 (mole fraction 18
Element has two or more isotopes that are used to determine its standard atomic weight. The isotopic abundances and standard 112 of isotope)
hydrogen atomic weights vary in normal materials. These variations are well known, and the standard atomic weight is given as lower atomic weight 112.414(4) helium
{
H 2 and upper bounds within square brackets, [ ]. uncertainty in last digit (112.414 ± 0.004) He
1 2 3
Element has two or more isotopes that are used to determine its standard atomic weight. The isotopic abundances and
1 atomic weights vary in normal materials, but upper and lower bounds of the standard atomic weight have not been assigned 4
[1.007 84, 1.008 11] 2 by IUPAC or the variations may be too small to affect the standard atomic weight value significantly. Thus, the standard 13 14 15 16 17 4.002 602(2)
lithium beryllium atomic weight is given as a single value with an uncertainty that includes both measurement uncertainty and uncertainty due boron carbon nitrogen oxygen fluorine neon
Li Be 9 to isotopic abundance variations. B C N O 17 F 19 Ne 2221
3 6 4 5 10 6 13 7 15 8 18 9 10
Element has only one isotope that is used to determine its standard atomic weight. Thus, the standard atomic weight is
7 invariant and is given as a single value with an IUPAC evaluated uncertainty. 11 12 14 16
9.0121831(5) [10.806, 10.821] [12.0096, 12.0116] [14.006 43, 14.007 28] [15.999 03, 15.999 77] 18.998403 163(6) 20
[6.938, 6.997] Element has no standard atomic weight because all of its isotopes are radioactive and, in normal materials, no isotope 20.1797(6)
sodium magnesium occurs with a characteristic isotopic abundance from which a standard atomic weight can be determined. aluminium silicon phosphorus sulfur 36 chlorine argon
(aluminum) 33 36
Na 23 Mg 26 27 Si 29 P 31 S 34 Cl Ar 38
11 12 25 Al 14 30 15 16 17 37 18
13 35
24 3 4 5 6 7 8 9 10 11 12 28 32 40
[24.304, 24.307] 26.9815385(7) [28.084, 28.086] 30.973761 998(5) [32.059, 32.076] [35.446, 35.457]
22.98976928(2) 39.948(1)
potassium calcium 48 scandium titanium vanadium chromium manganese iron cobalt nickel copper zinc gallium germanium arsenic selenium bromine krypton
40 4442 43 57 61 70 74 78
46 46 47 49 53 50 54 58 62 64 67 76 82 77 80
K 41 Ca Sc 45 Ti 50 V 50 Cr 54 Mn 55 Fe Co 59 Ni 60 Cu 65 Zn68 Ga 71 Ge70 73 As 75 Se76 Br 81 Kr 83
19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 82
66 78
40 48 51 52 56 58 63 64 69 72 74 80 79 86 84
39
40.078(4) 44.955908(5) 47.867(1) 50.9415(1) 51.9961(6) 54.938044(3) 55.845(2) 58.933194(4) 58.6934(4) 63.546(3) 65.38(2) 69.723(1) 72.630(8) 74.921595(6) 78.971(8) [79.901, 79.907]
39.0983(1) 83.798(2)
rubidium strontium yttrium zirconium niobium molybdenum technetium ruthenium rhodium palladium silver cadmium 108 indium tin antimony tellurium122123 iodine xenon 124
84 102 106 112114 124 130 128 126
86 87 89 92 91 93 97 94 99 100 96 103 Pd 104 109 113116 113 Sn 122 115 123 Te 120 127 Xe
Rb 87 Sr Y Zr 96 Nb Mo 98 Tc Ru 98 Rh 106 Ag Cd 114 In 124 120 Sb 125 130 I 136 132
37 38 39 40 41 42 100 43 44 45 46 110 47 48 49 50 117 51 52 126 53 54 134
94 110
92 101 119
105 111 131
85 88 90 95 96 104 102 107 112 115 116 121 128
87.62(1) 88.90584(2) 91.224(2) 92.90637(2) 95.95(1) 101.07(2) 102.90550(2) 106.42(1) 108 107.8682(2) 112.414(4) 114.818(1) 118.710(7) 118 121.760(1) 127.60(3) 126.90447(3) 129
85.4678(3) 131.293(6)
caesium barium 134 hafnium tantalum tungsten rhenium osmium iridium platinum gold mercury thallium lead bismuth polonium astatine radon
135 130 57 - 71 174 180 186184 192190 196
(cesium) 132 176 187 207
133 Ba136 Hf179 180 Ta 180 W 183 184 Re 185 Os188 192 Ir 191 Pt 198 Au 197 Hg198204 202 Tl 203 Pb 204 Bi 209 Po At Rn
56 137 lanthanoids 72 73 74 75 76 77 196 195 80
Cs 182 189 78 79 201 81 82 83 84 85 86
55 177 206
181 187 193 199 205 208
138 178 186 190 194 200
137.327(7) 178.49(2) 180.94788(2) 183.84(1) 186.207(1) 190.23(3) 192.217(3) 195.084(9) 196.966569(5) 200.592(3) [204.382, 204.385] 208.98040(1)
132.905451 96(6)
francium radium 89 - 103 rutherfordium dubnium seaborgium bohrium hassium meitnerium darmstadtium roentgenium copernicium ununtrium flerovium ununpentium livermorium ununseptium ununoctium
Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Fl Uup Lv Uus Uuo
87 88 actinoids 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118
lanthanum cerium praseodymium neodymium promethium samarium europium gadolinium terbium dysprosium holmium erbium thulium ytterbium lutetium
136138 144 154152 158 156 164162 168
La Ce 142 141 Nd148150 Sm150 Eu Gd 159 Dy 160 165 Er 169 Yb 170 Lu
138 Pr 142 Pm 148 152 151 155 158 Tb 161 164 Ho 170 166 Tm 176 174 176
57 58 59 60 145 61 62 63 64 65 66 67 68 69 70 71
143 157 171
149 167
163 175
139 146 144 147 154 153 156 160 173
138.905 47(7) 140.116(1) 140 140.90766(2) 144.242(3) 150.36(2) 151.964(1) 157.25(3) 158.92535(2) 162.500(1) 162 164.93033(2) 167.259(3) 168 168.93422(2) 173.054(5) 172 174.9668(1)
actinium thorium protactinium uranium neptunium plutonium americium curium berkelium californium einsteinium fermium mendelevium nobelium lawrencium
235 234
Ac Th 230 Pa 231 U Np Pu Am Cm Bk Cf Es Fm Md No Lr
89 90 91 92 93 94 95 96 97 98 99 100 101 102 103
232
238
232.0377(4) 231.035 88(2) 238.02891(3)
International Union of Pure and Applied Chemistry (IUPAC) Project 2007-038-3-200, "Development of an isotopic periodic table for the educational community" October 1, 2013 www.ciaaw.org
no reviews yet
Please Login to review.