283x Filetype XLSX File size 0.08 MB Source: research.unsw.edu.au
Sheet 1: NHMRC Project Grants 2016
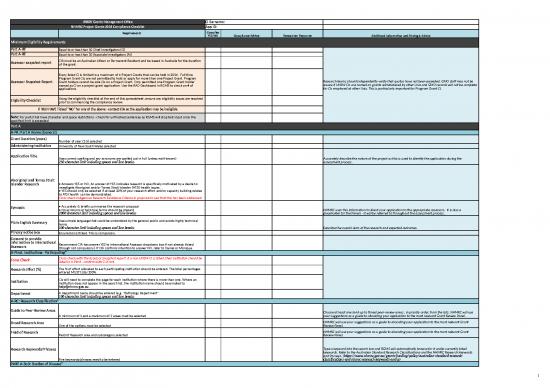
| UNSW Grants Management Office | CI Surname: | ||||
| NHMRC Project Grants 2016 Compliance Checklist | App ID: | ||||
| Requirements | Complies YES/NO | Compliance Advice | Researcher Response | Additional Information and Strategic Advice | |
| Minimum Eligibility Requirements | |||||
| Part A-RT | Equal to or less than 10 Chief Investigators (CI) | Research teams should independently verify that quotas have not been exceeded. GMO staff may not be aware if UNSW CIs are named on grants administered by other Unis and GMO records will not be complete for CIs employed at other Unis. This is particularly important for Program Grant CI. | |||
| Part A-RT | Equal to or less than 10 Associate Investigators (AI) | ||||
| Assessor snapshot report | CIA must be an Australian citizen or Permanent Resident and be based in Australia for the duration of the grant. | ||||
| Assessor Snapshot Report | Every listed CI is limited to a maximum of 6 Project Grants that can be held in 2016. Full time Program Grant CIs are not permitted to hold or apply for more than one Project Grant. Program Grant holders cannot be sole CIs on a Project Grant. Only permitted one Program Grant holder named as CI on a project grant application. Use the RAO Dashboard in RGMS to check on # of applications | ||||
| Eligibility Checklist | Using the eligibility checklist at the end of this spreadsheet, ensure any eligibility issues are resolved prior to commencing the compliance review. | ||||
| IF YOU HAVE Ticked "NO" for any of the above - contact CIA as the application may be ineligible | |||||
| Note: For parts that have character and space restrictions - check for unfinished sentences as RGMS will stop text input once the specified limit is exceeded | |||||
| Part A | |||||
| A-PA: Part A Home (General) | |||||
| Grant Duration (years) | Number of year (1-5) selected | ||||
| Administering Institution | University of New South Wales selected | ||||
| Application Title | Uses correct spelling and any acronyms are spelled out in full (unless well-known) 250 character limit including spaces and line breaks |
Accurately describe the nature of the project as this is used to identify the application during the assessment process. | |||
| Aboriginal and Torres Strait Islander Research | • Answers YES or NO. An answer of YES indicates research is specifically motivated by a desire to investigate Aboriginal and/or Torres Strait Islander (ATSI) health issues. • YES should only be selected if at least 20% of your research effort and/or capacity building relates to ATSI health can be demonstrated. Cross check Indigenous Research Excellence Criteria in proposal to see that this has been addressed. |
||||
| Synopsis | • Accurately & briefly summarise the research proposal • No acronyms or technical terms should be present 2000 character limit including spaces and line breaks |
NHMRC uses this information to direct your application to the appropriate assessors. It is also a placeholder for the Panels - it will be referred to throughout the assessment process. | |||
| Plain English Summary | Uses simple language that could be understood by the general public and avoids highly technical terms. 500 character limit including spaces and line breaks |
Describe the overall aims of the research and expected outcomes. | |||
| Privacy notice box | Ensure box is ticked. This is compulsory. | ||||
| Consent to provide information to International Assessors | Recommend CIA has answer YES to International Assessor dropdown box if not already ticked (though not compulsory). If CIA confirms intention to answer NO, refer to Daniel or Monique. | ||||
| A-PInst. Institutions- Participating* | |||||
| Cross Check | Cross-check with the Assessor Snapshot report: if a non-UNSW CI is listed, their institution should be listed in A-PInst - confirm with CI if not. | ||||
| Research Effort (%) | The % of effort allocated to each participating institution should be entered. The total percentages entered MUST total 100%. | ||||
| Institution | CIs will need to complete the page for each institution where there is more than one. Where an Institution does not appear in the search list, the institution name should be emailed to help@nhmrc.gov.au. | ||||
| Department | A department name should be entered (e.g. "Pathology Department". 100 character limit including spaces and line breaks |
||||
| A-RC: Research Classification* | |||||
| Guide to Peer-Review Areas | A minimum of 1 and a maximum of 3 areas must be selected | Choose at least one (and up to three) peer-review areas , in priority order, from the lists. NHMRC will use your suggestions as a guide to allocating your application to the most relevant Grant Review Panel. | |||
| Broad Research Area | One of the options must be selected | NHMRC will use your suggestions as a guide to allocating your application to the most relevant Grant Review Panel. | |||
| Field of Research | Field of Research area and subcategory selected | NHMRC will use your suggestions as a guide to allocating your application to the most relevant Grant Review Panel. | |||
| Research Keywords/Phrases | Five keywords/phrases need to be entered | Type a keyword into the search box and RGMS will automatically browse for it under currently listed keywords. Refer to the Australian Standard Research Classifications and the NHMRC Research Keywords and Phrases. (https://www.nhmrc.gov.au/grants-funding/policy/australian-standard-research-classifications-and-nhmrc-research-keywords-and-p) | |||
| PART A-BoD: Burden of Disease* | |||||
| Burden of Disease #1-#3 | (optional) up to 3 Burden of Disease types can be selected to best describe the area of research of the application. Ensure an allocation of time (%) has been given to each, and that allocation does not exceed 100%. If none have been entered, confirm with CIA that this is intentional. | The sum of the percentage (%) allocation must not exceed 100%. Click the SAVE button to calculate and display the total % allocation. | |||
| A-RT: Research Team and Commitment* | |||||
| Note: open each research team member and check all details for the below | Overseas based CIs and AIs cannot draw a salary from the budget. Salaries can be requested for all other personnel, including CIs A-J. Full salary can be requested if the time allocation for this project is 80% or greater. PSP levels should equate to the roles and responsibilities of the position rather than the expertise of a specific person. Various % PSPs can be requested. A limit of 10 CIs and 10 AIs can be included on any Project Grants Proposal | ||||
| Type | Correct roles assigned to project participants- Chief Investigator, Associate Investigator, Professional Research Person, Technical Support Staff A limit of 10 CIs and 10 AIs can be included on any Project Grants Proposal |
||||
| CIs | |||||
| Position Title | Does not need to be completed for CIs | CIs should be aware that the Qualifications & Skills section is pulled into the snapshot report and so a strong justification is required. It is not sufficient to simply provide a one sentence summary of the role. This will be the first impression of your track record to Assessors. PSP Level Requirements: • PSP1 - Technical support - non-graduate personnel • PSP2 - Junior graduate research assistant; or junior graduate nurse, midwife or allied health professional; or junior data manager/data analyst • PSP3 - Experienced graduate research assistant/junior postdoctoral research officer; or experienced graduate nurse, midwife or allied health professional; or experienced data manager/analyst • PSP4 - Experienced postdoctoral researcher (i.e., a researcher who may be considered as a named investigator on the research application and/or approaching the NHMRC CDF scheme or equivalent), or clinician without specialist qualifications • PSP5 - Senior experienced postdoctoral researcher (i.e., a researcher who would normally be considered as a named investigator on the research application and is more than 10yrs post-doctoral and/or would be expected to have applied for or held an NHMRC CDF (formerly CDA) or equivalent) |
|||
| Person | CIA has added the researcher to the proposal using their active RGMS account | ||||
| Role | CIA has allocated roles from list (CIB-CIJ) | ||||
| Australian Based | • CIs to select YES or NO • Ensure CIA has selected YES • CI B-J can select YES or NO, however all CIs drawing a salary from the grant MUST be based in Australia- cross check to ensure that no CIs who have selected NO (and are therefore based overseas) are requesting a salary for the grant. Requirements for CI salary support and to be based in Australia can be found under 7.2.3- 7.2.4 of the NHMRC Funding Rules (general), pages 7-8. |
||||
| Core Profile Data Entered | Must be 'YES'. If the 'yes' option is not available after selecting a participant, it means the core fields on his/her RGMS profile have not been populated, and you will not be able to add them as a CI on the team until this is complete. The CI will need to see the "Missing Profile Data" section on his/her RGMS home page to see which data fields need updating. | ||||
| Qualifications & Skills | Ensure each CIs participation is justified against specific qualifications or skills. 2000 character limit including spaces and line breaks For those also submitting a Cancer Australia application, includes the line "Chief Investigator A will be responsible for organising and managing the research collaboration and reporting to Cancer Australia and its Funding Partners" |
||||
| Proposed salary | Justification has been entered for PSP where requested |
||||
| Salary and Duration of Project | Ensure salary has only been allocated for the duration of the project (i.e. for a 3 year project, check no figures have been accidentally entered in Year 4 or Year 5) | ||||
| Career Disruption | Where CI selects 'YES', cross check with Grant Proposal to ensure Career Disruption page has been included for each CI where this is the case. | ||||
| AIs | |||||
| Position Title | • CIA may wish to include position titles in order to identify the roles performed by various Associate Investigators. | ||||
| Title, Name, Email | If the AI is not an existing RGMS account holder, check the following details have been manually entered: • Title • First, Middle & Last name • Email Address |
||||
| Professional Research Persons and Technical Support Staff | |||||
| Position Title | • CIA may wish to include position titles in order to identify a particular Professional Research Person (PRP) or Technical Support Staff (TSS) member. • Should not include the name of the PRP or TSS in the description of their role/title |
IMPORTANT- Requesting Salaries of PSP 4 and above: If you are requesting an PSP of Level 4 and above for a team member that will be supervised, you need to put forth a clear argument as to why an expert personnel would require supervision and why the role could not be performed by a team member recruited on a lower PSP. If you are requesting a PSP of Level 5, this person MUST be listed as a CI. Assessor comments consistently highlight failure to do so as a significant flaw in proposals. For detailed guidance on writing a PSP justification see: https://research.unsw.edu.au/document/writing_an_effective_psp_justification_2016.pdf |
|||
| Proposed salary | PSP has been chosen and justification entered. Important: PSP5 should not be selected for non-CIs. Refer CI to notes where this is the case. |
||||
| Salary and Duration of Project | Ensure salary has only been allocated for the duration of the project (i.e. for a 3 year project, check no figures have been accidentally entered in Year 4 or Year 5) | ||||
| PART A-EG: Ethics - General* | |||||
| NOTE | The Synopsis and Grant Proposal (B-PR) should be checked to see whether the research involves human participants or animal research, mentions stem cells or clinical trials, involves interviews, surveys, or other testing of people's reactions. This would indicate that ethics approval WOULD be required. Follow up with CIA where the former are present and the proposal indicates ethics approval not required. | If 'Yes' is answered to any of these questions, you will only need to obtain ethics approvals and supply evidence of these to the GMO after the project has been awarded. |
|||
| Ethics (Human) | Answers YES or NO to "Does this research proposal require submission to a human research ethics committee?" | ||||
| Ethics (Animal) | Answers YES or NO to "Does this research proposal require submission to an institution's animal ethics committee responsible for animal research?" | ||||
| Ethics (Other) | • Answers YES or NO to "Does this program involve organisms being genetically manipulated such that they fall under current guidelines issued by the Office of the Gene Technology Regulator?" • Answers YES or NO to "Does this program involve the use of carcinogenic or highly toxic chemicals?" |
||||
| Research Involving Stem Cells | • Answers YES or NO to "Will this research involve the use of human stem cells?" • Answers YES or NO to "Will this research involve the use of animal stem cells?" |
||||
| Part B | |||||
| PART B-AIProj: Application Information | |||||
| Career Disruption | CIA must select one of four options from drop-down box. If any career interruptions are being claimed, cross check part B-GP: Grant Proposal for the corresponding Career Disruption sections for each CI where applicable. • Each CI claiming a Career Disruption is permitted to add 1 page to the Grant Proposal describing the interruption. Check this page is only used to describe the career disruption, and does not include other factors related to the project/CI unrelated to career disruption. |
||||
| New Investigator | Applicant to select YES or NO. • A response of yes indicates that all CIs have already completed the NEW Investigator Eligibility Exemption request process, and have received a response from the NHMRC indicating their eligibility to apply as a New Investigator. • An application should not have 'YES' selected here if any CIs have not received a positive response on their New Investigator eligibility status from the NHMRC. |
Proof of NI statuses from NHMRC will be provided to UNSW in due course | |||
| Clinical Trial | CIA to select YES or NO. • Cross check an answer of 'NO' with the budget. If the budget lists things such as sites and study participants, then the project may in fact be a clinical trail and CIs should select 'YES'. If suspected, confirm with CIA as to whether this is a clinical trial. • Cross check an answer of 'YES' with the ethics section, to ensure human ethics approval has been ticked. This is required for all clinical trials. |
This question aims to identify applications that involve a clinical trial. | |||
| Funding Organisation | CIA to select the organisations they are submitting the proposal to (can be none, 1, 2 or all). If no box is selected, applications will be assessed by NHMRC only. • There is no obligation for 'NHMRC' to be selected- CIs are permitted to submit to the Cancer funding partners alone. • For applications being submitted only to the Cancer funding partners, the funding rules for these sponsors must be checked to determine CI eligibility- the NHMRC eligibility criteria do not apply. Confirm with CI that boxes have been ticked appropriately. |
The option to submit only to the Cancer funding partners allows researchers who will be (1) at the 6 Project Grant limit, or (2) on Program Grants to apply to these sponsors without infringing on NHMRC rulings in this regard. It is also an alternate source of funding for applications which might be considered 'fundable' by NHMRC but which may not be competitive within NHMRC. | |||
| Cancer Australia Young Investigator | Applicant to select YES or NO in response to "Investigator Is this application for Cancer Australia’s Priority-Driven Collaborative Cancer Research Young Investigator Initiative?" If YES, check that additional information has been provided in the research proposal (Part G) |
||||
| Note: If Applicable, see Cancer Australia & Cancer Council compliance spreadsheet on the additional tabs for feedback on supplementary applications to these schemes. | |||||
| Consent to disclose information to third parties | Ensure CIA has selected YES from the drop down box where you are applying to additional Organisations - If applying for Cancer Australia and/or Cancer Council, ensure these boxes are ticked. The 'Administering Institution/ Actual Institution' box must be ticked. | All CIs must consent and written evidence provided. Download the Investigator Endorsement template from CIs and AIs at https://research.unsw.edu.au/document/pg16_investigator_endorsement_final.docx. | |||
| Associate Investigator Permissions | Ensure CIA has selected YES where any AIs are included on the application, or N/A if none are included. | All AIs must consent and written evidence provided. Download the Investigator Endorsement template from CIs and AIs at https://research.unsw.edu.au/document/pg16_investigator_endorsement_final.docx. | |||
| PART B-GP: Grant Proposal (PDF Upload) | |||||
| Mandatory Template | Uses the NHMRC mandatory template. The template is available for download at https://research.unsw.edu.au/document/project_grants_grant_proposal_template_2016.doc | All formatting requirements are taken from the NHMRC Funding Rules Section 10.3.3 (pg. 18-19) Note: The NHMRC has sent eligibility letters to UNSW concerning proposals that have not adhered to these strict formatting requirements, and numerous Assessors have complained about the issue. Page Limits • Research Proposal- 9 pages total • References - 2 pages total • CI Time Commitment to this Proposal - 1/2 page per CI • Team Quality and Capability relevant to this proposal - 1 page total • CI Track Record, including the top 5 publications in the last 5 years - 2 pages per CI • Career Disruption - 1 page per CI, where applicable • Priority-driven Young Investigator - 1 page total, where applicable • Indigenous Research Excellence Criteria - 2 pages total, where applicable Notes on Colour Images: Colour diagrams, graphics and images may be included in the application, however researchers should keep in mind that PDFs are usually printed in black and white for distribution to the reviewing panel and there may be some loss of definition and colour in the images. |
|||
| PDF Format | The attachment is in PDF format Note: RGMS does not automatically convert a Word Template to PDF- the CIA must do this prior to uploading. |
||||
| PDF File size | PDF upload does not exceed 2MB in file size | ||||
| PDF Naming Convention | PDF file adheres to naming convention: [AppID]_[CIASurname]_Grant_Proposal.pdf • Spaces should not be included in the file name- use underscores only. |
||||
| Page Size | All pages must be A4 size | ||||
| Margins | Margins at least 2cm at the top/bottom and sides of the page. Note- the header and footer can be placed within this 2cm margin, but must be at least 1cm from top/bottom of page respectively. |
||||
| Header | Application ID & CIA Surname in top right corner; Title of the Page (Grant Proposal) top left corner. • Header must use size 10 Times New Roman font |
||||
| Footer | Page number to be included at bottom right corner • Footer must use size 10 Times New Roman font |
||||
| Section Headings | All section headings must use size 14 Times New Roman font and be bolded. | ||||
| Font | The main font in the body of the application must be size 12 Times New Roman | ||||
| Line Spacing | All line spacing must be set to single | ||||
| Character Spacing | All character spacing must be set to normal, with the scale set to 100% | ||||
| Diagrams, Graphics and Images | • Text that is part of an image is exempt from font and size requirements. • Description and or legends of all graphs and images must be no smaller than size 12 Times New Roman. • Labelling of graphs and images should be in a highly readable font such as Times New Roman or Ariel. |
Eligibility challenges have arisen in the past around this area - ensure all correct | |||
| Tables | Text in a table is not considered to be an image or diagram, therefore all text in tables must be size 12 Times New Roman. | ||||
| Web links | Does not include links to external websites, apart from references to published or peer reviewed journal articles that are only available online. Where links are included, provide the URL in full (e.g. NHMRC website - http://www.nhmrc.gov.au) Where journal web links are used, confirm with the CI that the journal is only available online |
||||
| A. Research Proposal | Includes the following headings, in order: • Aims (describes specific aims, includes clear statement of hypotheses to be tested) • Background (rationale for project) • Research Plan (details experiment design, techniques to be used, details and justification of controls, details for appropriate blinding, strategies for randomisation and/or stratification, justification of sample size, including power-calculation, justification of statistical methods, strategies to compensate for the effects of sex-differences, different animal strains and/or end points, ethical implications the research may have, community involvement and/or plans to transfer knowledge to stakeholders or into practice, expected outcomes of the project) • Timeline (detailed timeline for expected outcomes including justification for the duration requested) • Outcomes and Significance (describes importance of the problem to be researched, planned outcome of research plan and potential significance of research). |
A strong Research Proposal will lead with a strong proposition which is then supported by the Background (why THIS research, why now?) The Research Plan will be well thought through with methodology fully justified and alternative approaches rebutted as appropriate. Outcomes and significance will reference the opening proposition. The Research Strategy Office (RSO) has found that a lack of detail with regards to the statistical considerations of the experimental plan (for those proposing human, animal or genetic research) is one of the most frequent areas of assessor criticism. At a minimum, the Research Proposal should justify the numbers of subjects to be studied including consideration of power, effect size, drop-out and confounders. Pilot data ideally provide the basis for justifying these parameters. The RSO can provide further information on this topic. Contact nhmrc.rso@unsw.edu.au. The following useful guides, free on-line sample-size/power calculators are available as well as additional resources as per below: • Sample size calculator: http://www.stat.ubc.ca/~rollin/stats/ssize/ • AMC biostatistics manual: http://os1.amc.nl/mediawiki/images/Biostatistics_manual_sample_size.pdf • St George’s (University of London) “Statistics Guide for Research Applicants”: http://www-users.york.ac.uk/~mb55/guide/guide.htm o Checklist 1: http://www-users.york.ac.uk/~mb55/guide/checklst.htm o Checklist 2: http://www.ats.ucla.edu/stat/dae/ • The School of Public Health and Community Medicine may be offering paid consulting for grant applications and other biostatistical support- www.sphcm.med.unsw.edu.au |
|||
| Complies with the 9 page limit | |||||
| Additional Checks for project involving human or animal research of any kind (check whether CIA has answered YES to PART A-EG: Ethics) It is expected that a detailed statistical plan including justification for numbers of subjects, treatment of confounders, power, sample size and effect sizes as well as the proposed statistical analysis method should be included in the Proposed Research. Normally, this could be a stand-alone paragraph or be a shorter section accompanying each experimental section. Ensure these elements have been included. |
|||||
| B. References | Lists the References that are cited in the Background and Research Plan. | • Researchers should provide a list of all references cited in the application in an appropriate standard journal format, (NHMRC prefers the Author-date (also known as the Harvard System), Documentary-note and the Vancouver System) • List authors in the order they appear in PubMed • Only include references to cited work • Numbering can be used to refer to references |
|||
| Complies with the 2 page limit | |||||
| Does not include links to websites. | |||||
| Please note, the CI Time Commitment to this Proposal section has been removed by the NHMRC and should not be included in the final Grant proposal. | |||||
| C. Team Quality and Capability relevant to this proposal | Provides a summary of the research team's quality and capability, detailing: • the expertise and productivity of team members relevant to the proposed project; • their influence in this specific field of research; • how the team will work together to achieve the project aims; and • how junior members are contributing to the overall track record of the team. |
||||
| Complies with the 1 page limit | |||||
| D. CI track record, including the Top 5 publications in the last 5 years | Complies with the 2 page limit per CI | Why doesn’t NHMRC use impact factors/H-Index? • Journal rankings and journal impact factor (JIF) are journal-level metrics that describe the citation performance of the whole journal, not individual papers. • NHMRC is one of more than 400 organisations that have signed the Declaration on Research Assessment (DORA) which states “the impact factor must not be used as a surrogate measure of the quality of individual research articles, to assess an individual scientist’s contributions, or in hiring, promotion, or funding decisions” (Science 340,787 (2013). • The number of times that an individual paper is cited by another researcher is just one measure of the impact of a publication. For example, impact may well relate to health policy development, new clinical guidelines, decisions by health carers, or the development of new products and devices. Component 1- include information such as: Career summary (including qualifications, employment and appointment history); Research support (including grants and fellowships); Contribution to field of research (this may include the impact of previous research including translation of research into health outcomes); Patents (this information should include if the patent has been licensed, when they have been licensed, to whom they have been licensed and if that license is current or not); Collaborations; Community engagement and participation; Professional involvement (including committees, conference organisation, conference participation); International standing (including invitations to speak, international committees); Supervision and mentoring; Peer review involvement (including NHMRC, other granting organisations, manuscripts, editorial responsibilities);Other contributions to NHMRC; and any other information you think is vital to your application. |
|||
| Does not refer to an H-index of impact factors. Reference to H-indexes is not permitted for the Project Grants scheme. | |||||
| Ensure each CI on the proposal is mentioned in this section. Cross check against the Research Team section. | |||||
| Component 1: Overall Track Record in the last 5 years CIs have identifies aspects of their track record in addition to their publication record, including any relative to opportunity. |
|||||
| Component 2: The top 5 publications in the last 5 years CI lists 5 publications only- all must have been published within the last 5 years. Ensure an explanation as to 'WHY' chosen is provided |
|||||
| E. Career Disruption (where applicable) | Cross check YES has been ticked at B-AIProj and in A-RT | Career Disruption includes pregnancy; major illness; carer responsibilities including parental leave. Employment outside the research sector or in industry, clinical, administrative environments and/or teaching workload are NOT to be included in Career Disruption but should be included in the Track Record information more generally. These kinds of disruption will still be considered relative to opportunity. Claims for Sensitive Career Disruptions – 1 page per CI If a career disruption is of a highly sensitive nature and you (or members of your Team) do not wish to share this information in the Grant Proposal, applicants should: • Indicate in the Grant Proposal that they wish to make a claim under the career disruption provisions and that it is of a sensitive or private nature; • Complete a single page Career Disruption PDF (using the same format as above) . Ensure your application ID number is included in the PDF. Using the subject heading "NHMRC in–confidence", send the document to career.disruptions@nhmrc.gov.au, addressed "To the attention of the 2015 Project Grants funding scheme", by the application close date (5pm 18 March 2015) |
|||
| CI uses the Full time Equivalent table provided in the Grant proposal Template | |||||
| CI provides a brief summary (100-150 words) of the career disruption/s, including the duration and total, using the table provided in the Grant Proposal template | |||||
| CI details the impact on research output/productivity | |||||
| If relevant- CI provides details of additional research outputs (those that occurred in the relevant preceding years) | |||||
| If relevant- CI indicates any national or international conferences where they were invited to give a major presentation, or other significant invitations (e.g., to join an editorial board of a major journal, or write a major review), and were not able to do so because of considerations associated with the career disruption | |||||
| F. Priority-driven Young Investigator Project Grants | For those also applying for CA Young Investigator Check CIA is applying to NHMRC and Cancer Australia. |
||||
| Complies with the 1 page limit | |||||
| Includes the prescribed text as follows: This proposal is to be considered for funding from NHMRC and PdCCRS. Funding from NHMRC is sought for a project addressing the following aims: • Aim 1 • Aim 2 • Aim 3 etc. Funding from the PdCCRS is alternatively sought for the same project modified to one/two year/s. In the one/two year/s timeframe the project will only address the following aim/s: • Aim 1 • Aim 2 |
|||||
| G. Indigenous Research Excellence CriteriaS | CI describes and demonstrates that at least 20% of the research effort and/or capacity building activity will be directed to Aboriginal and/or Torres Strait Islander health | Full details on the Indigenous Research Criteria can be found at Section 6.2 of the NHMRC Funding Rules, and are pasted below: • Community engagement - the proposal demonstrates how the research and potential outcomes are a priority for Aboriginal and Torres Strait Islander communities with relevant community engagement by individuals, communities and/or organisations in conceptualisation, development and approval, data collection and management, analysis, report writing and dissemination of results. •Benefit - the potential health benefit of the project is demonstrated by addressing an important public health issue for Aboriginal and Torres Strait Islander peoples. This benefit can have a single focus or affect several areas, such as knowledge, finance and policy or quality of life. The benefit may be direct and immediate or it can be, indirect, gradual and considered. •Sustainability and transferability - the proposal demonstrates how the results of the project have the potential to lead to achievable and effective contributions to health gain for Aboriginal and Torres Strait Islander peoples, beyond the life of the project. This may be through sustainability in the project setting and/or transferability to other settings such as evidence-based practice and/or policy. In considering this issue the proposal should address the relationship between costs and benefits. •Building capability - the proposal demonstrates how Aboriginal and Torres Strait Islander peoples, communities and researchers will develop relevant capabilities through partnerships and participation in the project. |
|||
| CI addresses the Indigenous Research Excellence Criteria as set out in Section 6.2 of the NHMRC Funding Rules. These are: • Community engagement • Benefit • Sustainability and transferability • Building capacity |
|||||
| Complies with the 2 page limit | |||||
| PART B-PBRF: Proposed Budget - Research Facilities | |||||
| Cross-check proposal to see if there is a reference to use of a research facility. If YES, a letter may be uploaded (optional but adds to feasibility) | Note: that relates to third party only, not UNSW facilities | ||||
| Link to research facilities at: http://www.nhmrc.gov.au/research/national-research-facilities-and-networks | |||||
| PART B-PBRF: Proposed Budget - DRC and Equipment | |||||
| Direct Research Costs (DRCs) | No Personnel Support Packages requested under Direct Research Costs | All PSPs should be requested under A-RT: Research Team and Commitment | |||
| For each Direct Research Cost item, ensure the CI has provided: • the item name/description of the item - 50 character limit • the total value of the item requested for each year • a justification for the item which aligns with the aims of the study, be detailed on a yearly basis (i.e. provide details of how the item is required/used in each year) and be fully justified- 500 character limit including spaces & line breaks. |
Use of Research Facilities Any use of research facilities (such as ANFF or MWAC equipment) should be detailed under 'Direct Research Costs'. Ensure the budget amounts are accurate. Recent feedback has made clear that Assessors are cross-checking application budgets against costs posted on relevant faculty websites, and are very critical where costs do not align. Automatic Rounding to the nearest $5000 in RGMS: The total annual amount requested for each line item will be automatically rounded to the nearest $5,000 by the application form. The final rounded number is available at the ‘summary’ tab of the application form. |
||||
| No disallowed items have been requested, including: • Research infrastructure that an institution with research as a part of its mission would be expected to supply, institutional overheads and admin charges • Airline membership, health insurance and travel insurance • Entertainment costs, including Restaurant meals, alcohol and other hospitality • Standard computing, including personal computers, related peripherals or soft ware needed for communicating, writing and undertaking simple analyses. • Land, buildings and fixtures • Minor amounts of supplies, postage or telephone costs. To be classified as a DRC, the amount would have to be significantly greater than what an Admin Org would be expected to provide, and must be to support research activities, not administrative or clerical activities. |
The full NHMRC Direct Research Cost Guidelines can be found at: http://www.nhmrc.gov.au/_files_nhmrc/file/grants/funding/funded/manage/policy/drc_principles%20guidelines_1%20january_2014.pdf Allowable costs include but are not limited to: • Travel costs for a Research Activity, such as field work, research collaborations or for use of facilities in other countries • Conference costs related to the Research Activity or for presenting the outcomes of that Research Activity. • Refreshments for clinical trial participants • Specialised computing requirements • Publication costs |
||||
| Equipment | No Personnel Support Packages requested under Equipment | Researchers must detail why requests for equipment are specific to this particular project and will not be provided by UNSW. |
|||
| Total equipment budget does not exceed $80,000 | |||||
| No items of equipment requested under $10,000 | |||||
| For each item of equipment requested, CIA has provided a written quotation. UNSW will retain these quotes and make them available to the NHMRC on request. | |||||
| For each Equipment item, ensure the CI has provided: • the item name/description of the item - 50 character limit • the total value of the item requested for each year • a justification for the item which aligns with the aims of the study, be detailed on a yearly basis (i.e. provide details of how the item is required/used in each year) and be fully justified including why the equipment cannot be provided by the Institution)- 500 character limit including spaces & line breaks. |
|||||
| PART B-NPA: Nomination of Possible Assessors | |||||
| Nominating potential Assessors for this proposal- up to 2 National & 2 International | (Optional)- CIs are not required to nominate any Assessors, however by completing this section, this can assist the NHMRC in finding suitable Assessors for the proposal. Up to 2 National and 2 International assessors nominated |
Researchers are advised not to nominate any potential assessors who many have a conflict of interest with the application. This may include a previous or current collaboration, working within the same departments or a close personal relationship with the nominee. |
|||
| Where any national and/or international assessors are nominated, check details have been completed where the assessor is not an existing RGMS account holder (name, Expertise, Email, Phone, Fax) | |||||
| PART B-NA: Non Assessor | |||||
| Nominating a potential non-Assessor for this proposal | (Optional)- CIs are not required to nominate any non- Assessors, however are permitted to provide details of one person who they would not like to be approached to assess the application. | In providing justification for a non-assessor nomination, your reasons may include personal concerns which lead you to believe that the assessor would be incapable of giving a fair assessment due to unreasonable bias. Details of your requested non-assessor will be advised, in confidence, only to persons directly involved in the selection of the assessors of your application. |
|||
| Where any non-assessors are nominated, check details have been completed where the assessor is not an existing RGMS account holder (name, Expertise, Email, Phone, Fax) | |||||
| The following is only accessible from the "Assessor Snapshot Report" | |||||
| CV-PUB: | • Publications last 5 years. Publications held in open access repositories - researchers to tick yes or no. This is not a compliance issue - whilst the NHMRC mandates OA, there are situations where this is not always possible or the OA status will be forthcoming. • Check all CIs have something listed against their CV-PUB section. If nothing, it may mean they have not completed their profile in the RGMS CV section. |
||||
| CV-RF: | Check all CIs have something listed against their CV-RF section. If nothing, it may mean they have not completed their profile in the RGMS CV section. | ||||
| CV-ORF: | Check all CIs have something listed against their CV-ORF section. If nothing, it may mean they have not completed their profile in the RGMS CV section. | ||||
| UNSW GMO Requirements | |||||
| Investigator Endorsement - submitted by CIA as an email | AIs do not have access to the application in RGMS. They therefore are not required to endorse the application, but must provide written agreement to be listed. Download the Investigator Endorsement Email Template from https://research.unsw.edu.au/document/pg16_investigator_endorsement_final.docx | ||||
| Equipment quotes provided if required (to be retained by UNSW- no need to submit with the application) | |||||
| Supplementary Questions/Additional Questions/Consumer Review submitted if requesting funds from Cancer Australia, Cancer Council | |||||
| Use the following checklists within RGMS: | |||||
| App Checklist - the following items, if with a red cross means the application cannot be submitted | |||||
| A-PA: Part A Home - All pages on Part A are required | |||||
| A-RT: Research Team & Commitment. Is there a 'Nominated' CIA? | |||||
| A-RT: Research Team & Commitment (CI) | |||||
| A-RC: Research Classification | |||||
| A-EG: Ethics - General | |||||
| B-AIProj: Application Information | |||||
| B-PBRF: Proposed Budget - Research Facilities | |||||
| B-PB: Proposed Budget - DRC and Equipment | |||||
| A-PInst: Institutions - Participating | |||||
| B-GP: Grant Proposal | |||||
| These three are not compulsory and so a red cross may show: | |||||
| B-NPA: Nomination of Possible Assessors | |||||
| B-NA: Non Assessor | |||||
| A-BoD: Burden of Disease | |||||
| UNSW Grants Management Office | CIA: | ||||
| PG16 Cancer Council NSW Supplementary Spreadsheet | APP ID: | ||||
| Requirements | Complies YES/NO | Compliance Advice | Researcher Response | Additional Information and Strategic Advice | |
| Minimum Eligibility Requirements | |||||
| P&Cs p.1 | Confirm with CIA that the majority of research in this proposal will be conducted in NSW and the majority of costs are incurred in NSW. |
Note: collaborative projects with co-investigators located in other states and Territories will be eligible provided details of the proposed collaboration are clearly stated, giving geographical location of research, availability of other research assistance, etc. | |||
| P&Cs p.1 | Confirm with CIA that, if this is a collaborative project, the purchase of products or services in this proposal are directly related to the NSW component. | ||||
| P&Cs p.1-2 | Confirm with CIA that no CI on the proposal is a recipient of funds from the tobacco industry or organisations associated with the tobacco industry, including the Smoking and Health Research Foundation of Australia. No CI may be employed by a University that accepts funds from the tobacco industry for health related research or services (UNSW does not). | Research teams should independently verify that their teams and universities do not violate this rule. GMO staff will not be able to verify if UNSW CIs are named on grants administered by other Universities. | |||
| P&CS p.2 | No Cancer Council NSW Program Grant Chief Investigators are included on the proposal- Program Grant CIs are ineligible to apply as CIs for CCNSW Project Grants during the term of the Program Grant. | Recipients of a Cancer Council NSW Strategic Research Partnership Grant may simultaneously hold a Cancer Council NSW Project Grant for research not related to that funded under the Partnership agreement | |||
| ITA p. 1 & P&C p.2 | The proposal does not request more than the maximum funding limit of $120,000 per year for no more than three years. | ||||
| IF NO for any of the above - refer to Team Leader or GMO Director as application may be ineligible | |||||
| SUPPLEMENTARY QUESTIONS FORM | |||||
| Header | Application ID and CIA Surname in the top that header is correctly filled | ||||
| Font | Text is Time New Roman 12 | ||||
| Text size | Text is size 12 | ||||
| Q1- Chief Investigator A | Chief Investigator A - contact details are complete | ||||
| Q2- Administering Institution | UNSW details are correct as per below: Name of RAO: Daniel Owens, Director. Department: Grants Management Office. Institution: University of New South Wales. Address: UNSW. Suburb: Kensington. State: NSW. Postcode: 2052 Telephone No: 02 9385 7254 Email: mygrants.gmo@unsw.edu.au |
||||
| Q3- Scientific Title of your project | Complete, with "click here and type" text deleted | ||||
| Q4- Which Cancer Council are you applying to? | Cancer Council NSW has been selected | ||||
| Q5- Investigators | All information is complete for all CIs and AIs | ||||
| Q6- List of Investigators same as NHMRC form? | Ticked YES or NO | ||||
| If NO: Where CIA has included investigators on this CCNSW application that are not in the NHMRC application, check they have attached a one page Track Record document at the end of the application, which includes a list of publications for the last 5 years. | |||||
| Q7- Media Summary | Describes project in terms suitable for release to the media, briefly describing the overall aims of the research and expected outcomes in a manner the general public would understand. | ||||
| Does not use highly technical terms. | |||||
| Complies with the maximum 500 character limit | |||||
| Q8 - Relevance to Cancer | Includes information relevant to the causes, diagnosis, treatment or prevention of cancer, with a focus on short or medium term impacts on cancer. | ||||
| Complies with the maximum 1/2 page limit. | |||||
| Q9- Specific State criteria | N/A for CCNSW. Applicants has entered "N/A" | ||||
| Q10- Is your application also being considered for funding by the NHMRC and/or Cancer Australia? | Ticked YES or NO | ||||
| Q11- Scope of Research | Provide details of the modifications required to the project to fit the level of funding offered by Cancer Council | ||||
| Complies with the maximum 2 page limit. | |||||
| Q11.1- Scope of Research (Aims) | Provide details about the aims that will be retained and the aims that will be removed due to the reduced level of funding and/or reduced number of years | ||||
| Q11.2- Scope of Research (Timeline) | Provides a timeline showing six month measureable milestones. Ensure the timeline fits with the project duration and identified 6 monthly deliverables as below: Employment of staff, ethics approval, development of study measures, data collection, data analysis & manuscript preparation. |
||||
| Q11.3- Scope of Research (budget) | Discusses the changes to budget across the duration of the funding term | ||||
| Q12- Budget | Adheres to maximum funding limit of $120,000 per year for a maximum three years (CCNSW) | • Cancer Council NSW applicants are not restricted to requesting the NHMRC's PSP salary levels for grant-supported personnel. • Funds can be used to provide CIAs salary provided CIAs employment contract exceeds the duration of the Project Grant funding. |
|||
| Table provided in form is used | |||||
| Sufficient detail provided | |||||
| Q13- Research Support | Cancer Council Research Grants Held by any CIs 2011-2015- Filled in or N/A. RGMS publication reference used. | CIA should ensure this is correct. The GMO does not have access to data pertaining to CIs/AIs at other universities | |||
| Q14- Common Scientific Outline | Has marked all that apply in each category | ||||
| Q15- Ethics and Other Approvals | Has indicated where the research involves activities that would require ethics and other approvals | ||||
| CONSUMER REVIEW FORM | |||||
| Applicants should note that Consumer Review constitutes 50% of their application score. • The Consumer Review Form must be provided in lay language, and in a manner which can be read without reference to information submitted through RGMS, such that the Consumer Review Form can be understood by a non-researcher as a stand-alone document. The consumer review form is assessed by a consumer review panel that makes recommendations to the Cancer Council Board. The review panel are NOT academics. • Examples of successful answers on this form are provided in the Consumer Review Guidelines- http://www.cancercouncil.com.au/wp-content/uploads/2013/12/Consumer-Review-Guidelines-2016-FINAL.pdf |
|||||
| Submission requirements | Consumer Review Form is submitted to the GMO in word document form. The GMO will submit to the Cancer Council. | ||||
| Header | Application ID and Project Title in the top that header is correctly filled | ||||
| Page Limit | Complies with maximum 2 page limit | ||||
| Font | Uses Ariel font | ||||
| Text Size | Uses size 10 font | ||||
| Margins | Margins of original document have not been adjusted | ||||
| Instructions | Instructions deleted from beginning of form and within text boxes | ||||
| Q1- Extent of benefit | Explains how the results of the research will have an important positive impact on human lives | This section can include the following aspects: disease causation, prevention, diagnosis; treatment; physical and/or mental and/or social wellbeing; quality of life, dignity, and survival. | |||
| Q2- Pathway for realising the benefit | Provides a clear description of the steps required to reach the stated end benefits of the research | ||||
| Q3- Potential for application of findings | Explains how the research will be applied in the real world (over the short, medium or long term) | ||||
| Explains the barriers that will need to be addressed to be successful and how the CIs propose to address them | |||||
| Q4- Equity | Justifies the selection of the study sample and explains why CIs have included and excluded particular groups who could potentially benefit from the outcomes of this research | If relevant, CIs should outline how the proposal addresses an under-studied or under-served population and/or a population with a high burden of disease or poorer outcomes. | |||
| Q5- Consumer involvement | IMPORTANT: The review panel will not consider an application without demonstrated consumer review involvement. Outlines how relevant informed consumers have been involved during the development of the research proposal and the plan for ongoing consumer involvement in the research. |
• A consumer is a person affected by cancer as a survivor, family member or carer, or a representative from a group such as Cancer Voices NSW, Breast Cancer Action Group NSW, Consumers Health Forum, cancer support groups, organisational in-house consumer panels, etc. They must be named, trained and networked. A consumer is NOT a cancer patient, a member of your university ethics committee, or another researcher in your project. • Please see the Consumer Review Guidelines for further details. |
|||
| Explains how the consumer(s) discussed are ‘qualified’ to be involved. | |||||
no reviews yet
Please Login to review.