222x Filetype PDF File size 0.04 MB Source: farmasi.unhas.ac.id
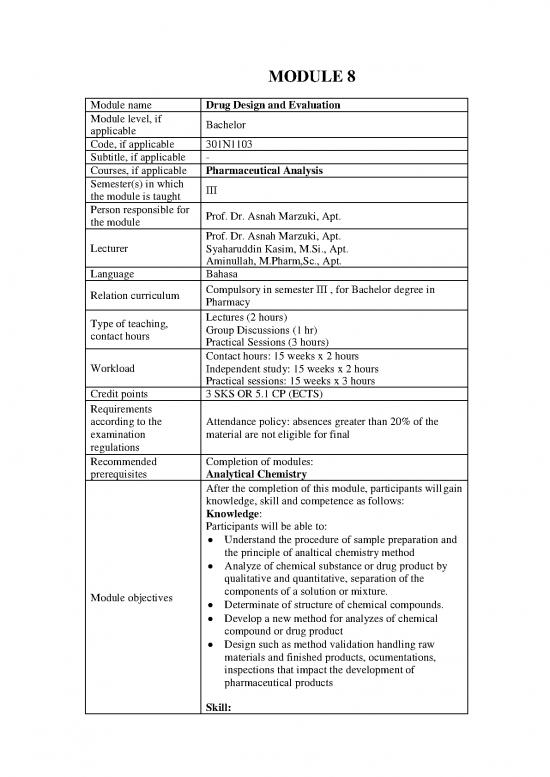
MODULE 8
Module name Drug Design and Evaluation
Module level, if Bachelor
applicable
Code, if applicable 301N1103
Subtitle, if applicable -
Courses, if applicable Pharmaceutical Analysis
Semester(s) in which III
the module is taught
Person responsible for Prof. Dr. Asnah Marzuki, Apt.
the module
Prof. Dr. Asnah Marzuki, Apt.
Lecturer Syaharuddin Kasim, M.Si., Apt.
Aminullah, M.Pharm,Sc., Apt.
Language Bahasa
Relation curriculum Compulsory in semester III , for Bachelor degree in
Pharmacy
Type of teaching, Lectures (2 hours)
contact hours Group Discussions (1 hr)
Practical Sessions (3 hours)
Contact hours: 15 weeks x 2 hours
Workload Independent study: 15 weeks x 2 hours
Practical sessions: 15 weeks x 3 hours
Credit points 3 SKS OR 5.1 CP (ECTS)
Requirements
according to the Attendance policy: absences greater than 20% of the
examination material are not eligible for final
regulations
Recommended Completion of modules:
prerequisites Analytical Chemistry
After the completion of this module, participants will gain
knowledge, skill and competence as follows:
Knowledge:
Participants will be able to:
• Understand the procedure of sample preparation and
the principle of analtical chemistry method
• Analyze of chemical substance or drug product by
qualitative and quantitative, separation of the
Module objectives components of a solution or mixture.
• Determinate of structure of chemical compounds.
• Develop a new method for analyzes of chemical
compound or drug product
• Design such as method validation handling raw
materials and finished products, ocumentations,
inspections that impact the development of
pharmaceutical products
Skill:
Participants will be able to understand the procedures, to
prepare of sample and using instruments for analyses
qualitative and quantitative of the chemistries using
several methods.
Competence:
Participants will be able to analyses qualitatively and
quantitatively of the chemistries using the right procedures
of the most suitable method.
This module is designed to develop material of Analytical
Chemistry. Previously, participants who have joined the
Analytical Chemistry materials are able to demonstrate
analysis of chemicals by using instrument with several
methods. This module is a further development.
Participants are equipped with material so as to perform
qualitative and quantitative chemical analysis whose
results can be accounted scientifically.
The laboratory sessions are designed to give further
understanding of the procedures of the most suitable
methods for sample preparation and
qualitative/quantitative analyses of chemistries.
Additional skill is given in how to understand the structure
of chemistries and their characteristics.
Content Topics covered may include:
▪ Basic methods and application of pharmaceutical
analysis
▪ Quantitative analysis of monocomponent and
multicomponent by spectrophotometry
▪ Quantitative analysis of monocomponent and
multicomponent by spectrofluorometry
▪ Analysis of drug by spectrophotometric derivatives
▪ Analysis of drug by TLC-Densitometry
▪ Analysis of drug by HPLC method
▪ Analysis of drug by Gas Chromatography method
▪ Analysis of analgesic, antipyretic and anti-
inflammatory drugs with several instruments
▪ Analysis of sulfonamide drug with several instruments
▪ Analysis of xanthine and barbiturates with several
instruments
▪ Analysis of antibiotic drug with several instruments
Students are marked based on their performance in theory
Study and examination (70%) and practicum (30%).
requirements and forms Performance in theory:
of examination • Final Exam (50%)
• Group discussion and presentation (40%)
• Individual quiz(10%)
Performance in practicum:
• Practical exam (25%)
• Preparation, assignments and quizzes (15%)
• Participation during experiments (30%)
• Discussion and reports (30%)
Lectures: slides, handouts, animation, white board
Group discussion: participants are divided in small
groups to address specific questions within the groups
before discussing them with the whole class. The process
is facilitated by lecturer(s).
Group presentation and posters: participants are divided
Media employed in small groups and given specific topic to be presented
using slides or posters in front of the class. The
presentation is marked by peers and lecturer(s).
Practicum: Participants are divided into small groups.
Experiments and assays are demonstrated by participants
based on practicum manuals and supervised by laboratory
assistants and lecturer(s).
David.G. Watson, Pharmaceutical Analysis: a textbook
for pharmacy students and pharmaceutical chemists,
Elsevier Publication, 2005.
Silverstein, R. M, Spectrometric Identification of Organic
Compounds, John Wiley and Sons, 2005.
,
Reading list Ewings, Analytical Instrumentation Handbook, 2005,
Third Edition. Florida Atlantic University Boca Raton,
Florida, USA.
Sudjadi, Rohman. Analisis Farmasi. Penerbit Pustaka
Pelajar.
Abdul Rohman. Analisis Obat. Gadjah Mada Universit
Press.
no reviews yet
Please Login to review.