243x Filetype PPTX File size 0.23 MB Source: www.wappingersschools.org
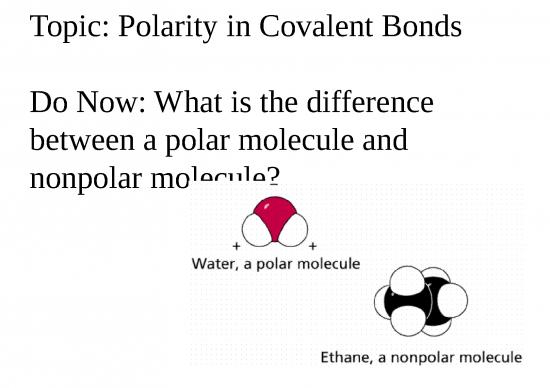
How do you tell Polar from Nonpolar??
• From the IMF unit we learned….
• If it’s dispersion (diatomic, noble
gas, pure hydrocarbon, small
symmetrical) it’s nonpolar
• If it’s not it’s polar
Polar Bond (Asymmetric)
• Polar – has poles
–Meaning ends are different
–Polar bond: one end more electrons
than other end
• So there is a Large difference in
electronegativity
Nonpolar Bond (symmetric)
• Nonpolar = No poles
• electron cloud on one end of bond same
as other end
• Same to no difference in electronegativity
H is symmetric. Both
2
ends are the same. The
electron cloud is
football-shaped.
HCl is asymmetric. The
electron cloud is lop-
sided. Chlorine has
more than its fair share.
Which bond(s) are polar?
Which are nonpolar?
Polar = LiH and HF. Nonpolar = H2
Red = electron rich.
Blue = electron poor.
no reviews yet
Please Login to review.