264x Filetype DOCX File size 0.12 MB Source: feinstein.northwell.edu
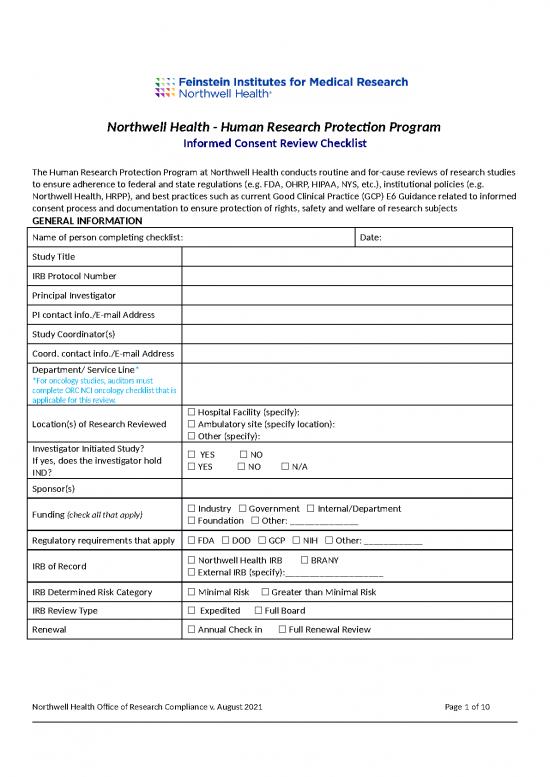
Northwell Health - Human Research Protection Program
Informed Consent Review Checklist
The Human Research Protection Program at Northwell Health conducts routine and for-cause reviews of research studies
to ensure adherence to federal and state regulations (e.g. FDA, OHRP, HIPAA, NYS, etc.), institutional policies (e.g.
Northwell Health, HRPP), and best practices such as current Good Clinical Practice (GCP) E6 Guidance related to informed
consent process and documentation to ensure protection of rights, safety and welfare of research subjects
GENERAL INFORMATION
Name of person completing checklist: Date:
Study Title
IRB Protocol Number
Principal Investigator
PI contact info./E-mail Address
Study Coordinator(s)
Coord. contact info./E-mail Address
Department/ Service Line*
*For oncology studies, auditors must
complete ORC NCI oncology checklist that is
applicable for this review.
☐ Hospital Facility (specify):
Location(s) of Research Reviewed ☐ Ambulatory site (specify location):
☐ Other (specify):
Investigator Initiated Study? ☐ YES ☐ NO
If yes, does the investigator hold ☐ YES ☐ NO ☐ N/A
IND?
Sponsor(s)
Funding (check all that apply) ☐ Industry ☐ Government ☐ Internal/Department
☐ Foundation ☐ Other: ______________
Regulatory requirements that apply ☐ FDA ☐ DOD ☐ GCP ☐ NIH ☐ Other: ____________
IRB of Record ☐ Northwell Health IRB ☐ BRANY
☐ External IRB (specify):____________________
IRB Determined Risk Category ☐ Minimal Risk ☐ Greater than Minimal Risk
IRB Review Type ☐ Expedited ☐ Full Board
Renewal ☐ Annual Check in ☐ Full Renewal Review
Northwell Health Office of Research Compliance v. August 2021 Page 1 of 10
IRB Initial Approval Date: ________
Study Information Institutional Approval Date: __________
First Subject Consent Date: ___________
Current IRB Expiration Date: __________
☐ Not Applicable
☐ (1) Research not involving greater than minimal risk (§45CFR46.404, §21CFR50.51).
☐ (2) Research involving greater than minimal risk, but presenting the prospect of direct benefit to the
individual subjects (§45CFR46.405, §21CFR50.52).
Pediatric Risk Category ☐ (3) Research involving greater than minimal risk and no prospect of direct benefit to individual subjects,
but likely to yield generalizable knowledge about the subject’s disorder or condition (§45CFR46.406,
§21CFR50.53).
☐ (4) 46.407 Research not otherwise approvable which present an opportunity to understand, prevent, or
alleviate a serious problem affecting the health or welfare of children (§45CFR46.407, §21CFR50.54).
Enrollment # (Enrolled is anyone who # IRB Approved: ________ # Enrolled to date: ________
signed an ICF)
1. STUDY PERSONNEL QUALIFICATIONS
NIH Policy NOT-OD-16-148: Policy on Good Clinical Practice Training for NIH Awardees Involved in NIH-funded Clinical Trials
GCP 2.8; 4.1.1; HRPP Policy 5.1 & 5.2: Principal Investigator & Research Team Members
Northwell Health Research Policy GR085: Training Requirements for the HRPP
Northwell Health Research Policy GR065: Review and Management of External Interests(COIs) in Research (Individual)
Northwell Health Research Policy GR097: IND/IDE Sponsor-Investigator Responsibilities
Completed HRPP Education
& Registration
Requirements?2 On File?
Annual COI
Can obtain CITI (HST & GCP (3yrs)), Date IRB Date IRB Disclosure License
# Name Role consent?1 COI (4yrs), etc.) approved removed Certified?3 CV: (If app)
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
1) Confirm training and the qualifications of each investigator who is authorized to obtain consent from subjects.
2) All individuals involved in research using biological agents must receive the appropriate level based on the type of research involved and
Northwell Health Office of Research Compliance v. August 2021 Page 2 of 10
should include those involved in the handling and management of biological agents.
3) For any investigators with positive COI disclosures requiring a management plan, specify any restrictions noted and review for compliance
with the plan (Check COI Smart).
Comments:
1. REGULATORY DOCUMENTATION & RECORD KEEPING
Reference: FDA 21CFR312.57 & 21CFR312.64; ICH GCP 4.9.4, 5.5.6, 5.5.7, & 8.1-8.4; Northwell Health Research Policy
GR042: Maintenance of Human Subject Research Regulatory Documentation
Depending on the type of study and sponsor, some studies require different regulatory documents. Review your
regulatory documents and complete this section according to the requirements that apply to your study.
YES NO N/A Reference:
2.1 Does the study team maintain regulatory documents? ☐ ☐
If so, how are regulatory documents maintained? (check all that apply)
2.2 ☐ Paper (e.g., regulatory binder)
☐ Electronically (e.g., shared file or system) ICH GCP 2.10, 4.9.4, 5.5.6, & 5.5.7
(If electronic)
2.3 Has the study team ensured all electronically filed ☐ ☐ ☐
documents are available and easily retrievable?
2.4 All CVs & licenses (if applicable) of key personnel on file? ☐ ☐
All licenses up-to-date? ICH GCP 4.1.1, 8.2.10 & 8.3.5
☐ ☐
Is there a subject screening/enrollment log? ☐ ☐
Is the subject screening/enrollment log complete? ☐ ☐
2.5 # of subjects included? _________ ICH GCP 8.3.20, 8.3.21,
8.3.22 & 8.4.3
# of subjects excluded? _________
Is there a subject identification code list? ☐ ☐
Is there an auditing/monitoring log? ☐ ☐
Was the log signed by the ORC auditor? ☐ ☐
2.6 Is the log complete/up to date? ☐ ☐ ICH GCP 8.3.10
If the study requires monitoring, how frequently
do reviews occur? ______________
Is there a staff signature log? ☐ ☐
Is staff signature log complete? ☐ ☐
2.7 Is there a staff delegation of responsibility log (can ☐ ☐ ICH GCP 4.1.5 & 8.3.24
be combined with staff signature log)?
Is the staff delegation log complete/accurate? ☐ ☐
(If applicable)
2.8 Are all versions of the Investigator’s Brochure or ☐ ☐ ☐
equivalent information on file? ICH GCP 7.1, 8.2.1 & 8.3.1
Product information sheet on file? ☐ ☐ ☐
2.9 Are lab tests required? ☐ ☐
2.10 If lab results are needed for: CLIA Public Law 100-578
Northwell Health Office of Research Compliance v. August 2021 Page 3 of 10
YES NO N/A Reference:
eligibility
treatment decisions based on test results
study results that are shared with subjects
treatment providers to alter care, New York State Public Health Law
Are labs done in a CLIA approved or NYS Dept. of Health Article 5, Title V
certified lab? ☐ ☐ ☐
2.11 Is a copy of normal lab values on file or the values are
documented along with the test results? ☐ ☐ ☐ ICH GCP 8.2.11 & 8.3.6
Is a copy of the lab certification on file or readily ☐ ☐ ☐
accessible? CLIA – CMS
2.12
(For research labs where applicable) Is a copy of the Lab ☐ ☐ ☐ ICH GCP 8.2.12 & 8.3.7
Director’s CV on file?
Is COI certification completed and up to date for each FDA 21CFR54; Northwell Health
2.13 ☐ ☐ Research Policy GR065: Conflicts of
investigator? Interest in Research
For a sponsor-investigator study, Northwell Health Research Policy
2.14 Is Form FDA 1572 ‘Statement of Investigator’ completed ☐ ☐ ☐ GR097: IND-IDE Sponsor-Investigator
and up to date for key study personnel? Responsibilities
2.15 All relevant correspondence to and from sponsor and/or
FDA on file (if app)? ☐ ☐ ☐ FDA 21CFR312; ICH GCP 4.9.4
Is this an applicable clinical trial or NIH funded ☐ ☐ US Public Law 110-85 (FDA
clinical trial? ☐ ☐ Amendments Act of 2007), Title VIII,
2.16 Is the study registered on clinicaltrials.gov? Sec.801
# NCT_______________________ ICMJE requirement
☐ ☐ ☐ Northwell Health Research Policy
Is the information up to date? GR020: Requirements for Registration
If the study is a clinical trial conducted/supported by a of Clinical Research at
2.17 Federal department/agency, does the study team have a ☐ ☐ ☐ www.clinicaltrials.gov
process/plan for posting a copy of the ICF to 45 CFR 46.116 (h) (1)
clinicaltrials.gov or regulations.gov?
Is there a Data Safety Monitoring Board/
Committee/ Plan for this study?
If yes, did they adhere to the plan? ☐ ☐ ☐ ICH GCP 6.8 & 8.3.18
2.18 Are all DSMB/C reports and recommendations on ☐ ☐ ☐
☐ ☐ ☐ HRPP Policy: Data and Safety
file? Monitoring
Have all DSMB/C reports been submitted to the ☐ ☐ ☐
IRB?
2.19 Does the study team have a plan for record retention upon
study completion? ☐ ☐ ☐ FDA 21CFR312.62
Please use this section to explain any issues with Regulatory Documentation & Record Keeping:
2. INSTITUTIONAL AND REGULATORY APPROVAL DOCUMENTATION- Relevant to Consent form and
Process Only
Reference: FDA 21CFR312; ICH GCP 4.4.1 & 4.5.1
Records YES NO NA Reference:
3.1 All relevant correspondence (e.g., submissions, ☐ ☐ FDA 21CFR312. 66
ICH GCP 4.4.3, 4.9.4, 8.3.3 & 8.3. 19
Northwell Health Office of Research Compliance v. August 2021 Page 4 of 10
no reviews yet
Please Login to review.