247x Filetype PDF File size 0.51 MB Source: www.gardencity.k12.ny.us
Name: ___________________________________________Date: ___________________ NOTES
Introduction to the Periodic Table, Metals, Nonmetals and Metalloids
Aim: How is the Periodic Table arranged and what patterns emerge among the elements?
Do Now: Please examine how the periodic table is arranged and with the person next to you, find as many
patterns as you can.
Periodic Law: ______________________________________________________________________________
__________________________________________________________________________________________
__________________________________________________________________________________________
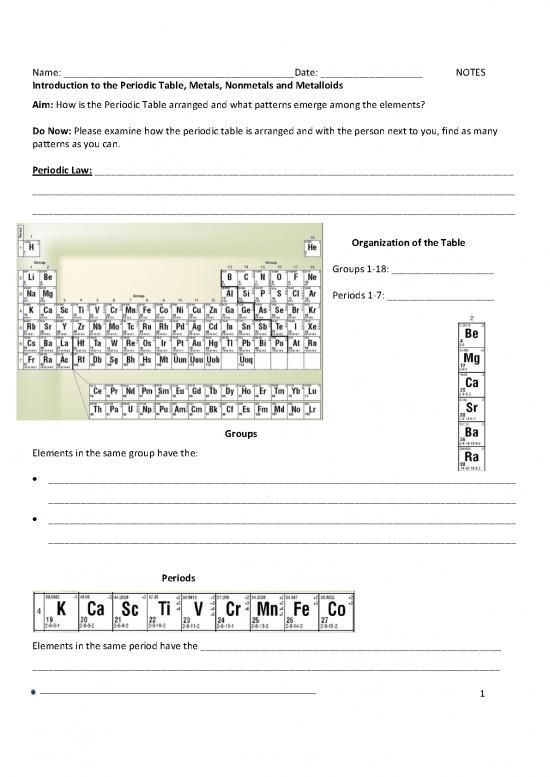
Organization of the Table
Groups 1-18: ___________________
Periods 1-7: ____________________
Groups
Elements in the same group have the:
• _______________________________________________________________________________________
_______________________________________________________________________________________
• _______________________________________________________________________________________
_______________________________________________________________________________________
Periods
Elements in the same period have the ________________________________________________________
_______________________________________________________________________________________
1
Name: ___________________________________________Date: ___________________ NOTES
Introduction to the Periodic Table, Metals, Nonmetals and Metalloids
Dmitri Mendeleev: Father of the Periodic Table
●Mendeleev organized the periodic table by listing elements in rows by increasing
atomic mass.
●He also put them in groups by reactivity.
Mendeleev’s table was close to the table we use today.
Mendeleev’s Table Modern Table
●Arranged elements by mass ● ____________________________________________
● Ordered elements in groups by ● ____________________________________________
reactivity ___________________________________________
The boron staircase is the dividing line for metals and nonmetals.
Metals, Nonmetals and Metalloids
• Metals_________________________________________________________________________________
_________________________________________________________
• ___________________________________. IT IS ONLY IN GROUP 1 BECAUSE IT
HAS 1 VALENCE ELECTRON.
• Nonmetals_________________________________________________________________________
__________________________________________________________________________________
• Metalloids _________________________________________________________________________
• Metalloid elements: ________________________________________
2
Name: ___________________________________________Date: ___________________ NOTES
Introduction to the Periodic Table, Metals, Nonmetals and Metalloids
Properties of Metals, Nonmetals and Metalloids
Aim: How do the properties of metals and nonmetals differ?
Metal Properties
All metals are solids except for mercury which is a liquid.
1._________________________________________________________
2._________________________________________________________
3._________________________________________________________
_________________________________________________________
4._________________________________________________________
_________________________________________________________
5. ________________________________________________________
Nonmetal Properties
The nonmetals exist as mostly gases, some solids and 1 liquid (Bromine)
1.__________________________________________________________
2.__________________________________________________________
3.__________________________________________________________
__________________________________________________________
4.__________________________________________________________
__________________________________________________________
5. __________________________________________________________
__________________________________________________________
Metalloid Properties
Metalloids can behave as a metal or a nonmetal, depending upon
what type of element they are reacting with.
1._________________________________________________________
2._________________________________________________________
3._________________________________________________________
4._________________________________________________________
5. ________________________________________________________
3
Name: ___________________________________________Date: ___________________ NOTES
Introduction to the Periodic Table, Metals, Nonmetals and Metalloids
Metallic and Nonmetallic Character
4
no reviews yet
Please Login to review.